Rapid tumor diagnosis enabling patient targeted cancer treatment
Summary
Tumour composition varies drastically, yet traditional diagnostic methods primarily target cancer cells, which comprise approximately 50% of the tumour. This narrow focus may result in an incomplete diagnostic perspective, thereby limiting the implementation of “Personalized Therapies” that could enhance treatment efficacy.
The new AI-Powered Diagnostic system developed by the Olivia Newton-John Cancer Research Institute (ONJCRI) is being leveraged to harness all information within the tumour, enabling a tailored treatment for each patient.
Currently in advanced development, this method is potentially suitable for 15 different tumour types. By integrating patented biosensor technology, it delivers reliable results in a cost-effective and time-efficient manner, with a processing time of approximately two hours. The system is user-friendly, requiring no specialized expertise to operate, and ensures reproducibility. Additionally, when deployed via cloud-based platforms, it becomes easily accessible to medical services in both urban and remote regions worldwide.
This funding call will focus on colorectal cancer (CRC) to enable a prototype as the first phase of proving the system’s effectiveness in this fast-increasing global disease.
Our story


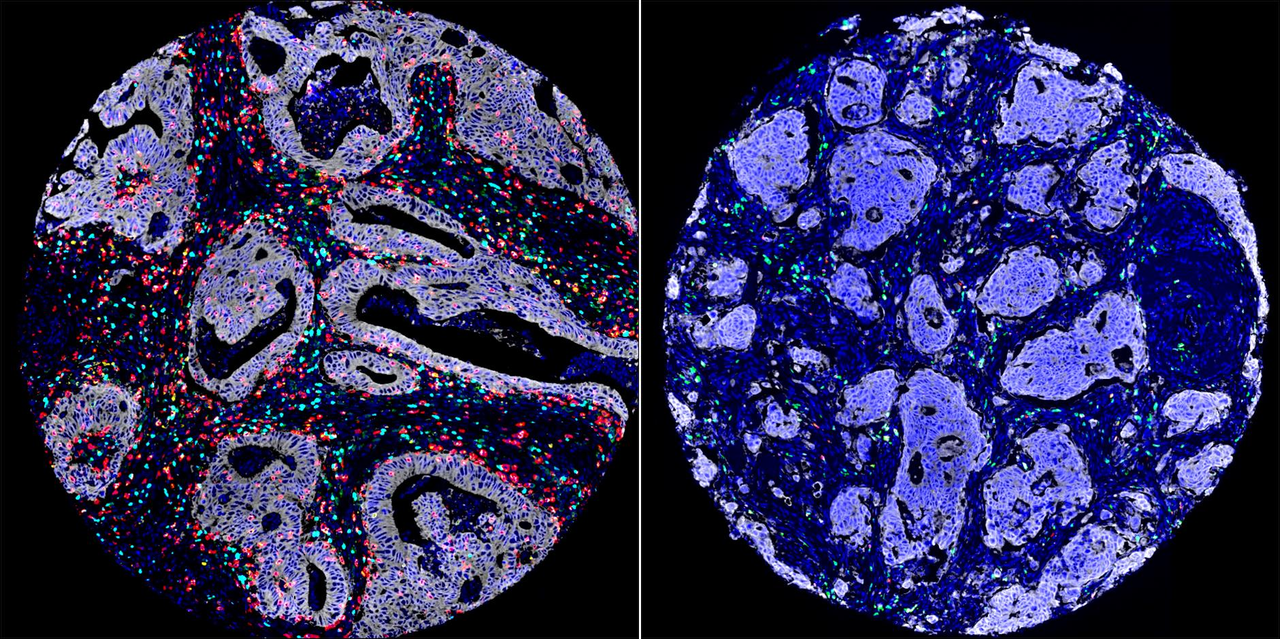
The method by which tumors are assessed makes a significant difference when developing personalized treatment approaches for patients on the individual level. Standard diagnostic methods review only the 50% portion of the tumour that has the cancer cell, a decision partly based on expediency and inability to standardise the process.
The other 50% of the tumor’ s ecosystem consists of immune cells (20%), the extracellular matrix — primarily collagen (20%) — and other cell types making up the remaining 10%. These components require different methods of analysis, which are often overlooked due to time and cost constraints. Unfortunately, focusing solely on cancer cells (50%) can result in significant differences in how patients respond to treatment, underscoring the limitations of traditional diagnostic systems.
There is evidence to show that assessment of tumor-infiltrating lymphocytes (TILs), immune cells within the tumour core, is a superior predictor of outcomes. TIL density and infiltration are significant predictors of survival in tumours. However, TILs assessment is not routinely used in clinical settings because of challenges in manual interpretation and the lack of cost-effective standardized method.
Digital pathology and AI offer a promising solution with fully automated digital imagine analysis. Developed as a collaboration between La Trobe University and the Olivia Newton-John Cancer Research Institute, the aim is to develop a robust prognostic and diagnostic approach using state-of-the art technologies.
Of the 15 cancer cell types that show potential for this method, the initial research and testing is being conducted on Colorectal cancer (CRC). As the third most diagnosed cancer there were 1.9 million cases reported and over 930,000 lives lost globally in 2023, figures that projected to triple in the next 15 years unless we are able to improve diagnosis and treatment.
Endorsed by
There is so much information as to why cancers evolve and what we can do about them. Until recently we haven’t been able to assimilate all this information into one coherent rule to understand the cancer better, or the likely means of managing it.
But with the new AI approaches suddenly there is an enormous opportunity to make good on all that information we are missing out on using. What these guys are doing has created an entirely new way of imaging which is cheap and efficient in ways we couldn’t conceive of even just two years ago.
With it we have both the analytical and the capturing capacity to put together in good, well annotated clinical data, and see what stories it can tell.

What makes this project interesting and why it works is that it makes cancer cells clearly visible on the basis of a colour change related to their optical refractive index properties. This is potentially much quicker and cheaper than current methods which use chemicals. With this new method, Brian's team have published research showing that cancer cells can be detected using a standard optical microscope without requiring any additional, often very expensive, equipment.
Brian originally worked with me as a post doctoral researcher many years ago during which time we undertook various X-Ray imaging projects. He then moved into the optical microscopy area, and since then I have followed his progress and collaborated in some of his research activities.
This technology is a huge leap ahead in the field of cancer diagnosis considering both the speed and the cost of perspectives, and enables cancer cells to be instantly seen with previously unprecedented clarity.
